Innovation
Current Research Directions
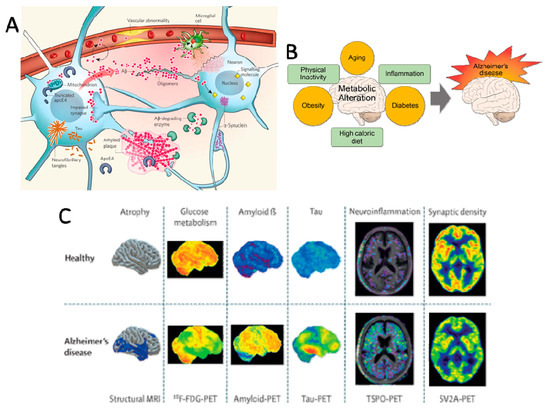
Scientists are exploring multiple approaches to better understand, treat, and potentially prevent Alzheimer's disease.
Amyloid-Targeting Therapies
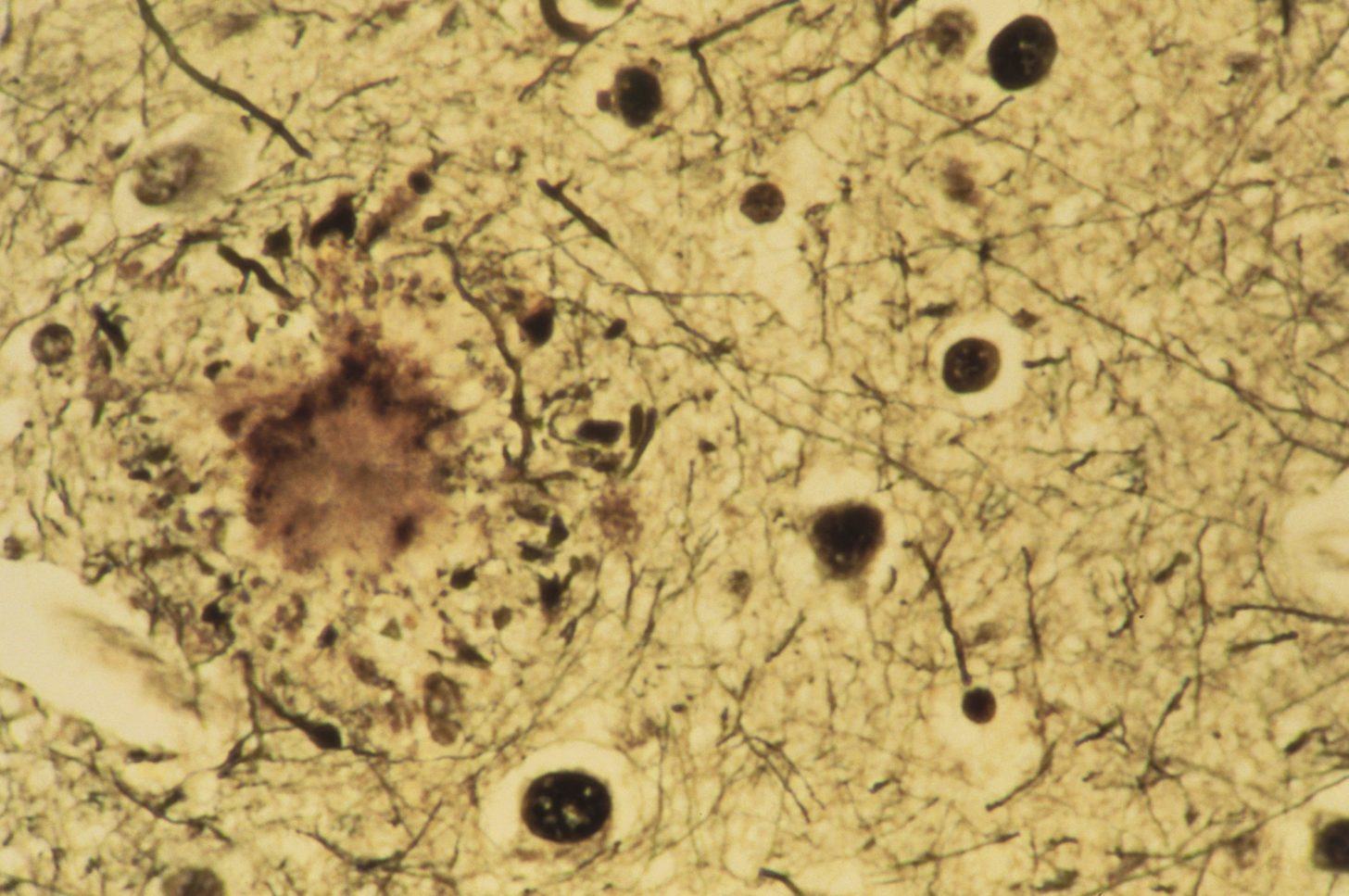
Beta-amyloid plaques are a hallmark of Alzheimer's disease. Researchers are developing several approaches to target these toxic protein deposits:
- Monoclonal Antibodies: These laboratory-produced molecules can bind to specific targets like amyloid plaques. Examples include aducanumab, lecanemab, and donanemab.
- Beta-secretase (BACE) Inhibitors: These drugs aim to block an enzyme involved in the production of beta-amyloid.
- Gamma-secretase Modulators: These compounds aim to alter the activity of another enzyme involved in amyloid production.
- Anti-aggregation Agents: These compounds aim to prevent beta-amyloid from clumping together to form plaques.
While some amyloid-targeting therapies have shown promise in clinical trials, the relationship between amyloid clearance and cognitive improvement remains complex and is an active area of research.